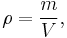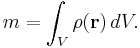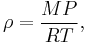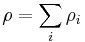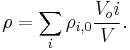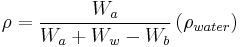Density
The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ (the Greek letter rho). In some cases (for instance, in the United States oil and gas industry), density is also defined as its weight per unit volume;[1] although, this quantity is more properly called specific weight. Different materials usually have different densities, so density is an important concept regarding buoyancy, purity and packaging. Osmium and iridium are the densest known metal elements at standard conditions for temperature and pressure but not the densest materials.
Less dense fluids float on more dense fluids if they do not mix. This concept can be extended, with some care, to less dense solids floating on more dense fluids. If the average density (including any air below the waterline) of an object is less than water (1000 kg/m3) it will float in water and if it is more than water's it will sink in water.
In some cases density is expressed as the dimensionless quantities specific gravity (SG) or relative density (RD), in which case it is expressed in multiples of the density of some other standard material, usually water or air/gas. (For example, a specific gravity less than one means that the substance floats in water.)
The mass density of a material varies with temperature and pressure. (The variance is typically small for solids and liquids and much greater for gasses.) Increasing the pressure on an object decreases the volume of the object and therefore increase its density. Increasing the temperature of a substance (with some exceptions) decreases its density by increasing the volume of that substance. In most materials, heating the bottom of a fluid results in convection of the heat from bottom to top of the fluid due to the decrease of the density of the heated fluid. This causes it to rise relative to more dense unheated material.
The reciprocal of the density of a substance is called its specific volume, a representation commonly used in thermodynamics. Density is an intensive property in that increasing the amount of a substance does not increase its density; rather it increases its mass.
Contents |
History
In a well-known but probably apocryphal tale, Archimedes was given the task of determining whether King Hiero's goldsmith was embezzling gold during the manufacture of a golden wreath dedicated to the gods and replacing it with another, cheaper alloy.[2] Archimedes knew that the irregularly shaped wreath could be crushed into a cube whose volume could be calculated easily and compared with the mass; but the king did not approve of this. Baffled, Archimedes took a relaxing immersion bath and observed from the rise of the water upon entering that he could calculate the volume of the gold wreath through the displacement of the water. Upon this discovery, he leaped from his bath and went running naked through the streets shouting, "Eureka! Eureka!" (Εύρηκα! Greek "I found it"). As a result, the term "eureka" entered common parlance and is used today to indicate a moment of enlightenment.
The story first appeared in written form in Vitruvius' books of architecture, two centuries after it supposedly took place.[3] Some scholars have doubted the accuracy of this tale, saying among other things that the method would have required precise measurements that would have been difficult to make at the time.[4][5]
Mathematically, density is defined as mass divided by volume:
where ρ is the density, m is the mass, and V is the volume. From this equation, mass density must have units of a unit of mass per unit of volume. As there are many units of mass and volume covering many different magnitudes there are a large number of units for mass density in use.
The SI unit of kilogram per cubic metre (kg/m³) and the cgs unit of gram per cubic centimetre (g/cm³) are probably the most common used units for density. (The cubic centimeter can be alternately called a millilitre or a cc.) 1000kg/m³ equals one g/cm³. In industry, other larger or smaller units of mass and or volume are often more practical and US customary units may be used. See below for a list of some of the most common units of density. Further, density may be expressed in terms of weight density (the weight of the material per unit volume) or as a ratio of the density with the density of a common material such as air or water.
Measurement of density
The density at any point of a homogeneous object equals its total mass divided by its total volume. The mass is normally measured with an appropriate scale or balance; the volume may be measured directly (from the geometry of the object) or by the displacement of a fluid. For determining the density of a liquid or a gas, a hydrometer or dasymeter may be used, respectively. Similarly, hydrostatic weighing uses the displacement of water due to a submerged object to determine the density of the object.
If the body is not homogeneous, then the density is a function of the position. In that case the density around any given location is determined by calculating the density of a small volume around that location. In the limit of an infinitesimal volume the density of an inhomogeneous object at a point becomes: ρ(r)=dm/dV, where dV is an elementary volume at position r. The mass of the body then can be expressed as
The density of granular material can be ambiguous, depending on exactly how its volume is defined, and this may cause confusion in measurement. A common example is sand: if it is gently poured into a container, the density will be low; if the same sand is then compacted, it will occupy less volume and consequently exhibit a greater density. This is because sand, like all powders and granular solids, contains a lot of air space in between individual grains. The density of the material including the air spaces is the bulk density, which differs significantly from the density of an individual grain of sand with no air included.
Changes of density
In general, density can be changed by changing either the pressure or the temperature. Increasing the pressure always increases the density of a material. Increasing the temperature generally decreases the density, but there are notable exceptions to this generalization. For example, the density of water increases between its melting point at 0 °C and 4 °C; similar behavior is observed in silicon at low temperatures.
The effect of pressure and temperature on the densities of liquids and solids is small. The compressibility for a typical liquid or solid is 10−6 bar−1 (1 bar=0.1 MPa) and a typical thermal expansivity is 10−5 K−1. This roughly translates into needing around ten thousand times atmospheric pressure to reduce the volume of a substance by one percent. (Although the pressures needed may be around a thousand times smaller for sandy soil and some clays.) A one percent expansion of volume typically requires a temperature increase on the order of thousands of degrees Celsius.
In contrast, the density of gases is strongly affected by pressure. The density of an ideal gas is
where M is the molar mass, P is the pressure, R is the universal gas constant, and T is the absolute temperature. This means that the density of an ideal gas can be doubled by doubling the pressure, or by halving the absolute temperature.
Density of water (at 1 atm)
| Temp (°C) | Density (kg/m3) |
|---|---|
| 100 | 958.4 |
| 80 | 971.8 |
| 60 | 983.2 |
| 40 | 992.2 |
| 30 | 995.6502 |
| 25 | 997.0479 |
| 22 | 997.7735 |
| 20 | 998.2071 |
| 15 | 999.1026 |
| 10 | 999.7026 |
| 4 | 999.9720 |
| 0 | 999.8395 |
| −10 | 998.117 |
| −20 | 993.547 |
| −30 | 983.854 |
| The density of water in kilograms per cubic metre (SI unit) at various temperatures in degrees Celsius. The values below 0 °C refer to supercooled water. |
|
Density of air (at 1 atm)
| T in °C | ρ in kg/m3 |
|---|---|
| –25 | 1.423 |
| –20 | 1.395 |
| –15 | 1.368 |
| –10 | 1.342 |
| –5 | 1.316 |
| 0 | 1.293 |
| 5 | 1.269 |
| 10 | 1.247 |
| 15 | 1.225 |
| 20 | 1.204 |
| 25 | 1.184 |
| 30 | 1.164 |
| 35 | 1.146 |
Density of solutions
The density of a solution is the sum of mass (massic) concentrations of the components of that solution.
Mass (massic) concentration of a given component ρi in a solution can be called partial density of that component.
Expressed as a function of the densities of pure components of the mixture and their volume participation, it reads:
Densities of various materials
| Material | ρ in kg/m3 | Notes |
|---|---|---|
| Interstellar medium | 10−25 − 10−15 | Assuming 90% H, 10% He; variable T |
| Earth's atmosphere | 1.2 | At sea level |
| Aerogel | 1 − 2 | |
| Styrofoam | 30 − 120[6] | |
| liquid hydrogen | 70 | |
| Cork | 220 − 260[6] | |
| Lithium | 535 | At STP |
| Potassium | 860[7] | At STP |
| Sodium | 970 | At STP |
| Ice | 916.7 | |
| Water (fresh) | 1000 | At STP |
| Water (salt) | 1030 | |
| Plastics | 850 − 1400 | For polypropylene and PETE/PVC |
| Magnesium | 1740 | At STP |
| Beryllium | 1850 | At STP |
| Glycerol[8] | 1261 | |
| Silicon | 2330 | At STP |
| Aluminium | 2700 | At STP |
| Diamond | 3500 | At STP |
| Titanium | 4540 | At STP |
| Selenium | 4800 | At STP |
| The Earth | 5515.3 | Mean density |
| Vanadium | 6100 | At STP |
| Antimony | 6690 | At STP |
| Zinc | 7000 | At STP |
| Chromium | 7200 | At STP |
| Manganese | 7210 - 7440 | At STP |
| Tin | 7310 | At STP |
| Iron | 7870 | At STP |
| Niobium | 8570 | At STP |
| Cadmium | 8650 | At STP |
| Cobalt | 8900 | At STP |
| Nickel | 8900 | At STP |
| Copper | 8920 − 8960 | Near room temperature |
| Bismuth | 9750 | At STP |
| Molybdenum | 10220 | At STP |
| Silver | 10500 | At STP |
| Lead | 11340 | Near room temperature |
| Thorium | 11700 | At STP |
| Rhodium | 12410 | At STP |
| The Inner Core of the Earth | ~13000 | As listed in Earth |
| Mercury | 13546 | At STP |
| Tantalum | 16600 | At STP |
| Uranium | 18800 | At STP |
| Tungsten | 19300 | At STP |
| Gold | 19320 | At STP |
| Plutonium | 19840 | At STP |
| Platinum | 21450 | At STP |
| Iridium | 22420 | At STP |
| Osmium | 22570 | At STP |
| The core of the Sun | ~150000 | |
| White dwarf star | 1 × 109[9] | |
| Atomic nuclei | 2.3 × 1017 [10] | Does not depend strongly on size of nucleus |
| Neutron star | 8.4 × 1016 − 1 × 1018 | |
| Black hole | 4 × 1017 | Mean density inside the Schwarzschild radius of an Earth-mass black hole (theoretical) |
Density of composite material
In the United States, ASTM specification D792-00[11] describes the steps to calculate the density of a composite material.
where:
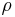 is the density of the composite material, in g/cm3
is the density of the composite material, in g/cm3
and
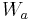 is the weight of the specimen when hung in the air
is the weight of the specimen when hung in the air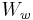 is the weight of the partly immersed wire holding the specimen
is the weight of the partly immersed wire holding the specimen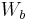 is the weight of the specimen when immersed fully in distilled water, along with the partly immersed wire holding the specimen
is the weight of the specimen when immersed fully in distilled water, along with the partly immersed wire holding the specimen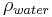 is the density in g/cm3 of the distilled water at testing temperature (for example 0.9975 g/cm3 at 23 °C)
is the density in g/cm3 of the distilled water at testing temperature (for example 0.9975 g/cm3 at 23 °C)
Other common units
The SI unit for density is:
- kilograms per cubic metre (kg/m³)
Litres and metric tons are not part of the SI, but are acceptable for use with it, leading to the following units:
- kilograms per litre (kg/L)
- grams per millilitre (g/mL)
- metric tons per cubic metre (t/m³)
Densities using the following metric units all have exactly the same numerical value, one thousandth of the value in (kg/m³). Liquid water has a density of about 1 kg/dm³, making any of these SI units numerically convenient to use as most solids and liquids have densities between 0.1 and 20 kg/dm³.
- kilograms per cubic decimetre (kg/dm³)
- grams per cubic centimetre (g/cc, gm/cc or g/cm³)
- megagrams per cubic metre (Mg/m³)
In U.S. customary units density can be stated in:
- Avoirdupois ounces per cubic inch (oz/cu in)
- Avoirdupois pounds per cubic inch (lb/cu in)
- pounds per cubic foot (lb/cu ft)
- pounds per cubic yard (lb/cu yd)
- pounds per U.S. liquid gallon (lb/gal)
- pounds per U.S. bushel (lb/bu)
- slugs per cubic foot.
In principle there are Imperial units different from the above as the Imperial gallon and bushel differ from the U.S. units, but in practice they are no longer used, though found in older documents. The density of precious metals could conceivably be based on Troy ounces and pounds, a possible cause of confusion.
See also
References
- ^ "Density definition in Oil Gas Glossary". Oilgasglossary.com. http://oilgasglossary.com/density.html. Retrieved 2010-09-14.
- ^ Archimedes, A Gold Thief and Buoyancy - by Larry "Harris" Taylor, Ph.D.
- ^ Vitruvius on Architecture, Book IX, paragraphs 9-12, translated into English and in the original Latin.
- ^ The first Eureka moment, Science 305: 1219, August 2004.
- ^ Fact or Fiction?: Archimedes Coined the Term "Eureka!" in the Bath, Scientific American, December 2006.
- ^ a b "Re: which is more bouyant [sic] styrofoam or cork". Madsci.org. http://www.madsci.org/posts/archives/mar2000/954534602.Ph.r.html. Retrieved 2010-09-14.
- ^ CRC Press Handbook of tables for Applied Engineering Science, 2nd Edition, 1976, Table 1-59
- ^ glycerol composition at physics.nist.gov
- ^ Extreme Stars: White Dwarfs & Neutron Stars, Jennifer Johnson, lecture notes, Astronomy 162, Ohio State University. Accessed on line May 3, 2007.
- ^ Nuclear Size and Density, HyperPhysics, Georgia State University. Accessed on line June 26, 2009.
- ^ (2004). Test Methods for Density and Specific Gravity (Relative Density) of Plastics by Displacement. ASTM Standard D792-00. Vol 81.01. American Society for Testing and Materials. West Conshohocken. PA.
External links
- Video: Density Experiment with Oil and Alcohol
- Video: Density Experiment with Whiskey and Water
- Glass Density Calculation - Calculation of the density of glass at room temperature and of glass melts at 1000 - 1400°C
- List of Elements of the Periodic Table - Sorted by Density
- Calculation of saturated liquid densities for some components
- field density test
- On-line calculator for densities and partial molar volumes of aqueous solutions of some common electrolytes and their mixtures, at temperatures up to 323.15 K.
- Water - Density and Specific Weight
- Temperature dependence of the density of water - Conversions of density units
- A delicious density experiment
- Water density calculator Water density for a given salinity and temperature.
- Liquid density calculator Select a liquid from the list and calculate density as a function of temperature.
- Gas density calculator Calculate density of a gas for as a function of temperature and pressure.
- Densities of various materials.